X

> ARTICLES
Injectable HIV PrEP: A new era of HIV prevention
Written by Yeva Margaryan, MD | Published on February 03, 2024
Reviewed by Slava Fuzayloff
Table of Contents
What is HIV PrEP?
PrEP (pre-exposure prophylaxis) is a medication that helps to prevent HIV acquisition among HIV-negative, high-risk individuals, for example, injectable drug users, those who have multiple sex partners, or those whose partner is HIV-positive.If a person takes PrEP as prescribed, he/she can reduce the risk of contracting HIV by up to 99%. [1] Pre-Exposure Prophylaxis (PrEP). Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/hiv/risk/prep/index.html#:~:text=Pre%2Dexposure%20prophylaxis%20(or%20PrEP,use%20by%20at%20least%2074%25
Types of PrEP
There are two types of PrEP: oral and injectable.
Oral PrEP
To date, two pills are approved for oral PrEP:
✓ Truvada®. Reduces the risk of HIV transmission through injectable drug use and sex
✓ Descovy®. Reduces the risk of HIV transmission through sex among those who are assigned male sex at birth (cisgender men and transgender women)
Injectable PrEP
Apretude is the only drug approved for injectable PrEP and is recommended for people who are at risk of acquiring HIV through sexual contact. [2] About PrEP. Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/hiv/basics/prep/about-prep.html
PrEP Injection
A long-acting, injectable PrEP (Aperture) has recently been approved as a more convenient alternative to daily oral tablets. It is a medication administered through an intramuscular (IM) injection once in two months.
The active ingredient of this injectable PrEP is Cabotegravir; it blocks the action of a specific enzyme (integrase) responsible for integrating HIV genetic material into human DNA, thereby preventing its replication and development of disease. Cabotegravir has been long used as part of HIV treatment (combination antiretroviral therapy (ART)). [3] Injectable long acting antiretroviral for HIV treatment and prevention: perspectives of potential users. BMC Infectious Diseases. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9936705/
Effectiveness
According to research, Injectable PrEP has at least 99% effectiveness at preventing HIV transmission through sex. [4] What You Need to Know About the New HIV Prevention Medication Apretude. PrEPdaily. https://prepdaily.org/what-you-need-to-know-about-the-new-hiv-prevention-medication-apretude/#:~:text=They%20are%20between%2074%2D84,more%20effective%20than%20PrEP%20medication
As of today, there is no data on the effectiveness of injectable PrEP among injectable drug users. Therefore, PrEP shots are not recommended for this population. However, research suggests that injectable PrEP may be a better option for those injectable drug users who fail to take oral PrEP daily or who are unable to store the PrEP pills. Also, injectable PrEP can be an option for those who inject drugs and are also at high risk of HIV acquisition through sex (e.g., those who have multiple sex partners or have an HIV-positive partner). [5] Injectable PrEP Frequently Asked Questions – Clinical. California Prevention Training Center. https://californiaptc.com/prep-learning-collaboratives/long-acting-injectable-prep/injectable-prep-frequently-asked-questions/
Baseline assessment for injectable PrEP
Prior to initiating injectable PrEP, it is essentialto make sure the person is not infected with HIV. The following are recommendations for HIV testing for different groups of people:
✓
An HIV antibody test should be performed among patients who have not had recent (last 4 weeks) potential exposure to HIV (e.g., unprotected sex with a partner of unknown HIV status) or recent symptoms of acute HIV infection (e.g., skin rash, fever, fatigue, mouth ulcers and etc.).
[6, 7]
Apretude package insert. U.S. Food and Drug Administration. (FDA).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
What is Injectable HIV Prep. Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/stophivtogether/library/topics/prevention/brochures/cdc-lsht-prevention-brochure-clinicians-quick-guide-what-is-injectable-hiv-prep.pdf
✓
If a person had recent potential exposure to HIV or had symptoms of acute HIV infection, an HIV RNA test should be performed to detect primary HIV infection.
[6, 7]
Apretude package insert. U.S. Food and Drug Administration. (FDA).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
What is Injectable HIV Prep. Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/stophivtogether/library/topics/prevention/brochures/cdc-lsht-prevention-brochure-clinicians-quick-guide-what-is-injectable-hiv-prep.pdf
✓
An HIV antibody or RNA test is also required for those who have taken post-exposure prophylaxis (PEP) or oral PrEP in the past 3 months or received injectable PrEP in the past 12 months.
[6, 7]
Apretude package insert. U.S. Food and Drug Administration. (FDA).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
What is Injectable HIV Prep. Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/stophivtogether/library/topics/prevention/brochures/cdc-lsht-prevention-brochure-clinicians-quick-guide-what-is-injectable-hiv-prep.pdf
Candidates for injectable PrEP are also recommended to be screened for STIs (chlamydia, syphilis, and gonorrhea).
Routine assessment for injectable PrEP
Patients should be assessed for acute HIV signs and symptoms and get tested for HIV prior to each PrEP shot. In addition, MSM (bisexual, gay, and other men who have sex with men) and transgender women who have sex with men should be screened for STIs at least every 4 months (starting with the 3rd injection). In heterosexually active patients, STI screening should be performed every 6 months (starting with the 5th injection).
[6, 7]
Apretude package insert. U.S. Food and Drug Administration. (FDA).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
What is Injectable HIV Prep. Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/stophivtogether/library/topics/prevention/brochures/cdc-lsht-prevention-brochure-clinicians-quick-guide-what-is-injectable-hiv-prep.pdf
If HIV infection is confirmed, the patient should be switched to an HIV treatment regimen. [1] Apretude package insert. U.S. Food and Drug Administration. (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
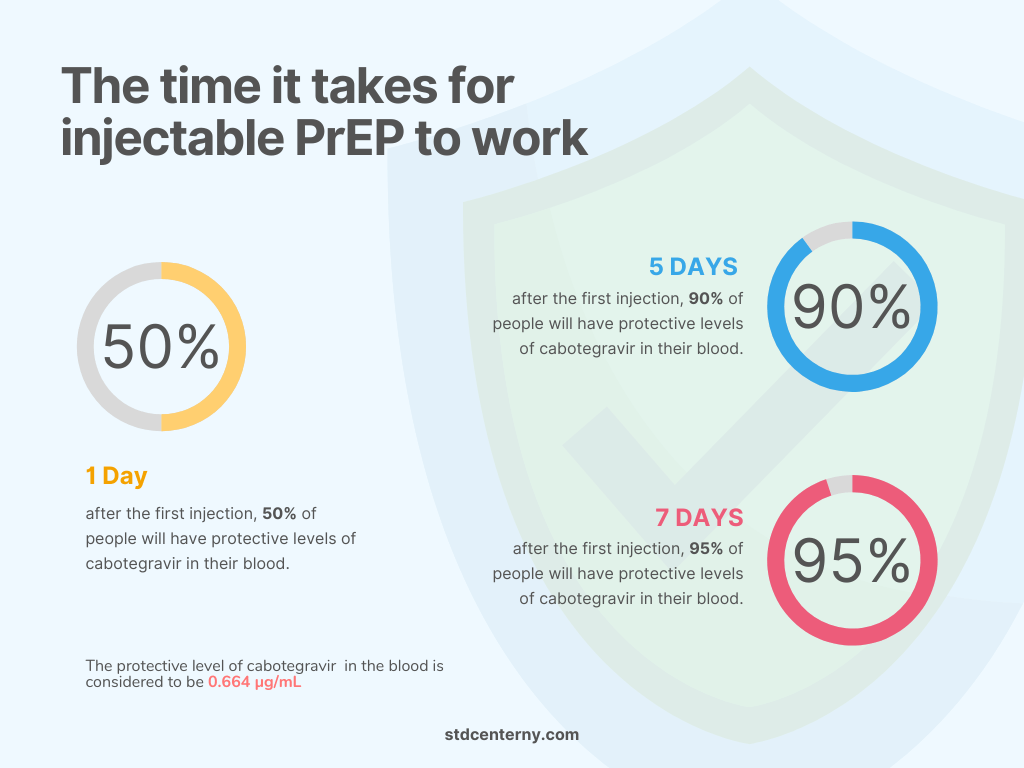
How long does it take to work?
The time required to achieve protection after injectable PrEP has not yet been established. However, available data from animal studies suggests that: [5] Injectable PrEP Frequently Asked Questions – Clinical. California Prevention Training Center. https://californiaptc.com/prep-learning-collaboratives/long-acting-injectable-prep/injectable-prep-frequently-asked-questions/
✓ 95% of people will have a protective concentration (0.664 μg/mL) of cabotegravir after a week of the first injection.
✓ 90% of people will have protective blood levels of the cabotegravir after three days of the first PrEP injection
✓ 50% of people will have protective levels of cabotegravir in their blood within a day of their first PrEP shot.
How to stop injectable PrEP?
There are important recommendations to consider when deciding to stop injectable PrEP:
[7, 8]
What is Injectable HIV Prep. Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/stophivtogether/library/topics/prevention/brochures/cdc-lsht-prevention-brochure-clinicians-quick-guide-what-is-injectable-hiv-prep.pdf
Long-Acting Injectable PrEP is Here:Frequently Asked Questions (FAQs) forImplementation. NASTAD.
https://getprepbroward.com/documents/Long-Acting-Injectable-PrEP.pdf
✓ Those who remain at potential risk of contracting HIV through sexual contact or injection drug use are strongly advised to switch to daily oral PrEP within eight weeks after their last PrEP shot.
✓ Patients are advised to visit their doctor quarterly for twelve months and get HIV RNA and antigen/antibody tests at each visit.
In any case, PrEP injections can be restarted at any time in the future, provided that the person is confirmed to be HIV-negative. [8] Long-Acting Injectable PrEP is Here:Frequently Asked Questions (FAQs) forImplementation. NASTAD. https://getprepbroward.com/documents/Long-Acting-Injectable-PrEP.pdf
Please note:
The active ingredient in injectable PrEP (cabotegravir) can remain in the body for several years (3 and 4 years for men and women, respectively) after the last injection. If a person becomes infected with HIV during this period (the so-called pharmacokinetic (PK) “tail”), he or she may develop resistance to cabotegravir, which will significantly complicate the selection of an HIV treatment regimen.
[7, 8]
What is Injectable HIV Prep. Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/stophivtogether/library/topics/prevention/brochures/cdc-lsht-prevention-brochure-clinicians-quick-guide-what-is-injectable-hiv-prep.pdf
Long-Acting Injectable PrEP is Here:Frequently Asked Questions (FAQs) forImplementation. NASTAD.
https://getprepbroward.com/documents/Long-Acting-Injectable-PrEP.pdf
Injectable PrEP schedule
How timely should the injection be?
There is a slight difference between the schedule of the first PrEP injections and the consecutive ones:
✓ First twoinjections: Injectable PrEP should begin with an intramuscular injection of 600 mg (3 ml) of cabotegravir, followed by a second dose given one month apart.
✓ Consecutive injections: After the first two doses of injectable PrEP are given sequentially one month apart, intramuscular (IM) injections of cabotegravir 600 mg (3 ml) should be given every two months.
Before getting the first PrEP injection, your healthcare provider might tell you to get one cabotegravir tablet orally every day for one month (at least 28 days) to evaluate your tolerance to cabotegravir.If all goes well, injections should be given on the last day or within three days after stopping the oral course of cabotegravir. [6] Apretude package insert. U.S. Food and Drug Administration. (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
What if I do not get it on time?
It is strongly recommended to follow the Injectable PrEP dosing schedule. However, sometimes, people unintentionally forget about an upcoming injection or deliberately decide to discontinue the further injection. Either way, here are the guidelines for both scenarios: [6] Apretude package insert. U.S. Food and Drug Administration. (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
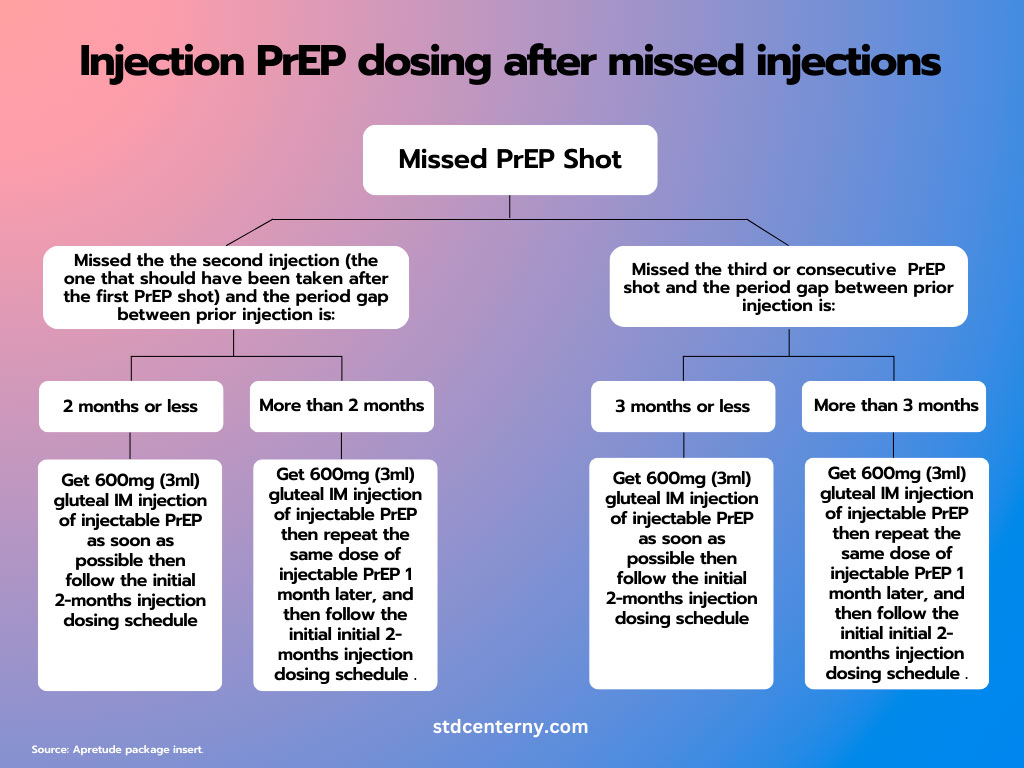
Planned Missed Injections
If a person plans to skip the scheduled every 2-month continuation PrEP shot by more than 7 days, he/she should replace that one missed injection with daily oral cabotegravir (30mg tablet) taken for up to 2 months. If the gap between two PrEPinjections will be longer than these two months, an alternative oral PrEP regimen should be considered. A person should start taking oral PrEP approximately two months after their last PrEP injection. [6] Apretude package insert. U.S. Food and Drug Administration. (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
PrEP injections can be resumed on the last oral PrEP dose or within three days. [6] Apretude package insert. U.S. Food and Drug Administration. (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
Unplanned Missed Injections
If a person unintentionally misses or delays a PrEP injection for more than seven days and has not taken oral PrEP in the interim, he/she should be reassessed for injectable PrEP to ensure that he/she is still eligible for injections. [6] Apretude package insert. U.S. Food and Drug Administration. (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
Dosing recommendations for PrEP shots after planned or unplanned missed injections are summarized in the algorithm below. [6] Apretude package insert. U.S. Food and Drug Administration. (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
Injection PrEP dosing after missed injections
How strict is the timing?
Receiving injectable PrEP as scheduled is a must! The PrEP injection can be taken up to 7 days before or after the scheduled injection. If a person misses a scheduled PrEP shot by more than seven days but is still at high risk of acquiring HIV, it is strongly recommended to take alternative PrEP medications to prevent potential HIV infection. [6] Apretude package insert. U.S. Food and Drug Administration. (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
Please note: Missed or delayed injections and failure to adhere to an every-2-monthly injection schedule can result in HIV infection and the development of resistance to HIV treatment. [6] Apretude package insert. U.S. Food and Drug Administration. (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
Switching from oral PrEP to Injectable PrEP
How soon after starting the injectable PrEP can I stop taking oral PrEP?
Based on the available evidence, oral PrEP can be stopped 7 days after the first PrEP shot. This is how long it takes approximately 95% of people to receive adequate protection against HIV infection.
[5, 6]
Injectable PrEP Frequently Asked Questions – Clinical. California Prevention Training Center.
https://californiaptc.com/prep-learning-collaboratives/long-acting-injectable-prep/injectable-prep-frequently-asked-questions/
Apretude package insert. U.S. Food and Drug Administration. (FDA).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
Is it worthwhile to switch to injection PrEP?
There are several facts to consider when thinking about switching from oral PrEP to injectable PrEP: [2] About PrEP. Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/hiv/basics/prep/about-prep.html
✓ Injectable PrEP is not recommended for people who are at risk of getting HIV via injectable drug use
✓ Injectable PrEP is costly (see Cost comparison for injection PreP vs. oral PrEP section)
✓ A person should visit health care provider to get each PrEP shot
✓ HIV testing prior to each shot is a must
However, injectable PrEP can significantly increase adherence to PrEP and be more practical among those who fail to take daily pills. Adherence, in turn, is a central factor in the effectiveness and success of PrEP.
Who to switch?
PrEP shots will be a great option for those who: [7] What is Injectable HIV Prep. Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/stophivtogether/library/topics/prevention/brochures/cdc-lsht-prevention-brochure-clinicians-quick-guide-what-is-injectable-hiv-prep.pdf
✓ Find it hard to take oral PrEP pills every day and instead prefer to take one injection every 2 months.
✓ With severe kidney disease contradicting the use of PrEP pills
Importantly, patients should weigh at least 77 lbs (35 kg), have confirmed HIV-negative status,and have no history of intolerance to cabotegravir to be eligible for PrEP injections.
Can I be switched to oral prep if I want? How to do it?
Yes. It is possible to switch from injectable PrEP to oral PrEP, but you should consult with your health care provider first to make a final decision based on your specific health factors and preferences. Anyways, it is important to initiate the oral PrEP within 2 months of the last PrEP shot (ideally a week before the next scheduled PrEP injection). [5] Injectable PrEP Frequently Asked Questions – Clinical. California Prevention Training Center. https://californiaptc.com/prep-learning-collaboratives/long-acting-injectable-prep/injectable-prep-frequently-asked-questions/
Oral PrEP vs. Injectable PrEP
Effectiveness comparison for injection PrEP vs. oral PrEP
Evidence suggests that PrEP shots are more effective in reducing the likelihood of HIV acquisition among MSM and transgender women than oral PrEP.
[8, 12]
Long-Acting Injectable PrEP is Here:Frequently Asked Questions (FAQs) forImplementation. NASTAD.
https://getprepbroward.com/documents/Long-Acting-Injectable-PrEP.pdf
Injectable pre-exposure prophylaxis is better than oral form in preventing HIV infection among MSM, trans women. UCLA Health.
https://www.uclahealth.org/news/injectable-pre-exposure-prophylaxis-better-oral-form
Two clinical trials were conducted to compare the effectiveness of injectable PrEP (with oral cabotegravir lead-in) among HIV-negative men and transgender women(Trial 1) and uninfected cisgender women (Trial 2). Both groups were at high risk of contracting HIV. Injectable PrEP has been shown to be more effective in HIV prevention in both trials, with a 69% and 90% lower risk of acquiring HIV compared with oral PrEP in Trial 1 and Trial 2, respectively. [9] FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention. U.S. Food and Drug Administration. (FDA). https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention
There is no conclusive evidence on the effectiveness of PrEP shots among injectable drug users. Whereas oral PrEP reduces the risk of HIV infection among people who inject drugs by approximately 74%. However, injectable PrEP may still be a good option for those drug users who do not adhere to the daily schedule of oral PrEP.
[1, 5]
Pre-Exposure Prophylaxis (PrEP). Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/hiv/risk/prep/index.html#:~:text=Pre%2Dexposure%20prophylaxis%20(or%20PrEP,use%20by%20at%20least%2074%25
Injectable PrEP Frequently Asked Questions – Clinical. California Prevention Training Center.
https://californiaptc.com/prep-learning-collaboratives/long-acting-injectable-prep/injectable-prep-frequently-asked-questions/
Side effects comparison for injection PrEP vs. oral PrEP
People taking injectable PrEP are more likely to report side effects than those taking oral PrEP. Common side effects include injection site reactions (for PrEP shots and placebo injections during trials), fever, headache, fatigue, rash, and muscle pain. More details on comparing the side effects of injectable and oral PrEPare provided below. [9] FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention. U.S. Food and Drug Administration. (FDA). https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention
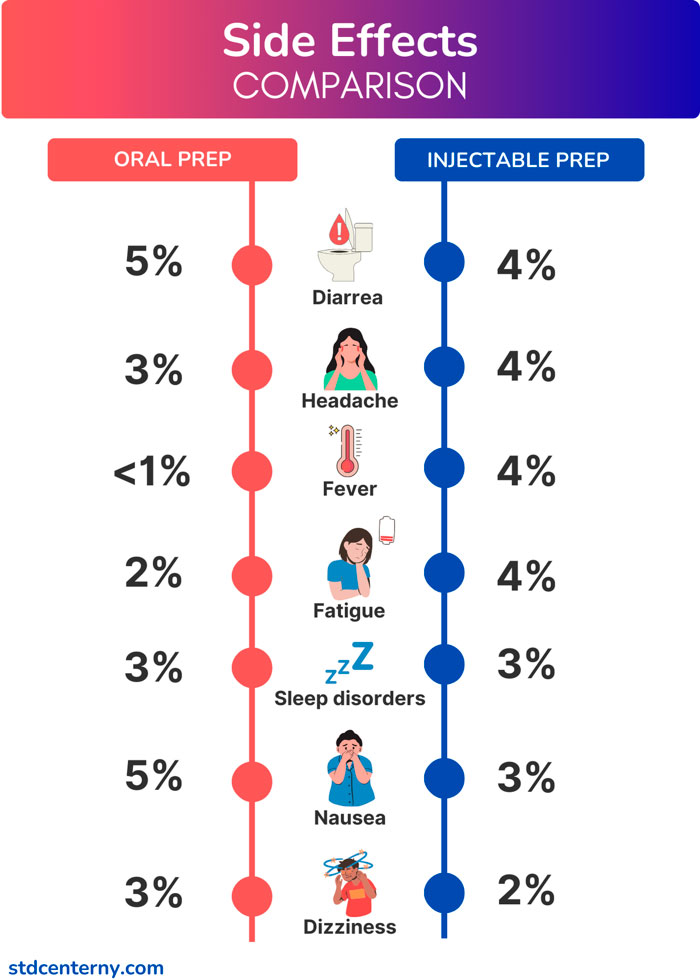
Injection site reactions are the most common side effect among people receiving injectable PrEP (82% of cases). These reactions last on average 8 days and decrease in severity with each subsequent injection. These side effects include but are not limited to local pain, swelling, induration, redness, and bruising.
Taking over-the-counter pain relievers before (or immediately after) the injection or applying a heating pad (or warm compresses) to the injection site for 15 to 20 minutes post-injection can significantly ease injection site reactions.
[5, 6]
Injectable PrEP Frequently Asked Questions – Clinical. California Prevention Training Center.
https://californiaptc.com/prep-learning-collaboratives/long-acting-injectable-prep/injectable-prep-frequently-asked-questions/
Apretude package insert. U.S. Food and Drug Administration. (FDA).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
Weight gain comparison for injection PrEP vs. oral PrEP
Studies have shown that patients receiving injectable PrEP gain slightly more weight (1.23 kg/ 2.7 lbs per year) compared to those receiving oral PrEP (Truvada®) (0.37 kg/0.8 lbs per year). [10] Injectable PrEP.The Australasia Society for HIV, Viral Hepatitis, and Sexual Health Medicine (ASHM). https://prepguidelines.com.au/goals-of-prep/injectable-prep/
Convenience comparison for injection PrEPvs. oral PrEP
In general, injectable PrEP is more convenient because it is long-acting and requires only one injection every 2 months. So, with injectable PrEP, a person gets only 6 doses a year, compared to 365 doses with oral PrEP. Many users of oral PrEP complain that they find it challenging to adhere to their daily medication regimen: it is simply neither desirable nor practical. In addition, injectable PrEP is known to reduce the stigma around PrEP use and its association with HIV risk behaviors. However, receiving injectable PrEP requires visiting a health facility and being tested for HIV each time, which requires time and resources.
[9, 12]
FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention. U.S. Food and Drug Administration. (FDA).
https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention
Injectable pre-exposure prophylaxis is better than oral form in preventing HIV infection among MSM, trans women. UCLA Health.
https://www.uclahealth.org/news/injectable-pre-exposure-prophylaxis-better-oral-form
Cost comparison for injection PreP vs.oral PrEP
As of December 29, 2022, a single dose (3 ml) of injectable PrEP costs $3,700 in the U.S., making the annual cost of injectable PrEP approximately $22,000 ($25,900 for the first year since the second shot is given 1 month after the first shot instead of 2-months period for each consecutive shot). This is about 60 times the price of oral PrEP (generic oral pills cost $30-$60 per month). Moreover, since PrEP injections should be administered by a health care provider, extra costs such as office visits and administration costs may apply.
[8, 11, 13]
What is Injectable HIV Prep. Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/stophivtogether/library/topics/prevention/brochures/cdc-lsht-prevention-brochure-clinicians-quick-guide-what-is-injectable-hiv-prep.pdf
Long-acting injectable cabotegravir for PrEP: A game-changer in HIV prevention? HIV Medicine.
https://onlinelibrary.wiley.com/doi/full/10.1111/hiv.13451
How Much Does Truvada for PrEPCost?. WebMD.
https://www.webmd.com/hiv-aids/how-much-truvada-for-prep-costs
Apretude for PrEP
FDA approval
Apretude is a long-acting injectable form of cabotegravir used as injectable PrEP. It was approved by the US Food and Drug Administration (FDA) in December 2021 and is still the first and only drug approved for PrEP injections.
[6, 8]
Apretude package insert. U.S. Food and Drug Administration. (FDA).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
Long-Acting Injectable PrEP is Here:Frequently Asked Questions (FAQs) forImplementation. NASTAD.
https://getprepbroward.com/documents/Long-Acting-Injectable-PrEP.pdf
Contradictions
Apretude is contradicted to: [6] Apretude package insert. U.S. Food and Drug Administration. (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf
✓ HIV-positive patients (or those with unknown HIV status)
✓ Those with a history of hypersensitivity reaction to cabotegravir
✓ People receiving the following drugs:
✓ Anticonvulsants, such as Arbamazepine, Phenytoin, Oxcarbazepine, or Phenobarbital
✓ Antimycobacterials, such as Rifampin, Rifapentine
The abovementioned drugs may reduce the level of cabotegravir in the blood and affect its effectiveness in preventing HIV infection.
Will insurance cover Apretude administration?
Apretude is likely to be covered as a medical benefit because it is administered in a medical facility and/or as a pharmacy benefit. Copayment terms are based on how it will be covered. You can talk to your health care provider for more information on your specific case. [8] Long-Acting Injectable PrEP is Here:Frequently Asked Questions (FAQs) forImplementation. NASTAD. https://getprepbroward.com/documents/Long-Acting-Injectable-PrEP.pdf
What if I don’t have insurance?
Individuals without insurance can get Apretude through the ViiVConnect program provided that they meet all criteria mentioned below: [8] Long-Acting Injectable PrEP is Here:Frequently Asked Questions (FAQs) forImplementation. NASTAD. https://getprepbroward.com/documents/Long-Acting-Injectable-PrEP.pdf
✓ Are a resident of one of the fifty states, Puerto Rico, or the District of Columbia.
✓ Cannot be a Medicaid beneficiary.
✓ Their household income does not exceed 500% of the federal poverty level.
In addition to the abovementioned conditions, one of the following criteria must also be met: [8] Long-Acting Injectable PrEP is Here:Frequently Asked Questions (FAQs) forImplementation. NASTAD. https://getprepbroward.com/documents/Long-Acting-Injectable-PrEP.pdf
✓ A person does not have prescription drug coverage
✓ Is a Medicare part B or part D beneficiary and made out-of-pocket payments of $600 or more for prescription drugs during that calendar year.
✓ Have a private insurance plan that is limited to either:
✓ Outpatient use only, or
✓ Generic-only coverage, or
✓ Therapeutic class exclusion
Source
-
Pre-Exposure Prophylaxis (PrEP). Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/hiv/risk/prep/index.html#:~:text=Pre%2Dexposure%20prophylaxis%20(or%20PrEP,use%20by%20at%20least%2074%25 -
About PrEP. Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/hiv/basics/prep/about-prep.html -
Injectable long acting antiretroviral for HIV treatment and prevention: perspectives of potential users. BMC Infectious Diseases.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9936705/ -
What You Need to Know About the New HIV Prevention Medication Apretude. PrEPdaily.
https://prepdaily.org/what-you-need-to-know-about-the-new-hiv-prevention-medication-apretude/#:~:text=They%20are%20between%2074%2D84,more%20effective%20than%20PrEP%20medication -
Injectable PrEP Frequently Asked Questions – Clinical. California Prevention Training Center.
https://californiaptc.com/prep-learning-collaboratives/long-acting-injectable-prep/injectable-prep-frequently-asked-questions/ -
Apretude package insert. U.S. Food and Drug Administration. (FDA).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf -
What is Injectable HIV Prep. Centers for Disease Control and Prevention (CDC).
https://www.cdc.gov/stophivtogether/library/topics/prevention/brochures/cdc-lsht-prevention-brochure-clinicians-quick-guide-what-is-injectable-hiv-prep.pdf -
Long-Acting Injectable PrEP is Here:Frequently Asked Questions (FAQs) forImplementation. NASTAD.
https://getprepbroward.com/documents/Long-Acting-Injectable-PrEP.pdf -
FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention. U.S. Food and Drug Administration. (FDA).
https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention -
Injectable PrEP.The Australasia Society for HIV, Viral Hepatitis, and Sexual Health Medicine (ASHM).
https://prepguidelines.com.au/goals-of-prep/injectable-prep/ -
Long-acting injectable cabotegravir for PrEP: A game-changer in HIV prevention? HIV Medicine.
https://onlinelibrary.wiley.com/doi/full/10.1111/hiv.13451 -
Injectable pre-exposure prophylaxis is better than oral form in preventing HIV infection among MSM, trans women. UCLA Health.
https://www.uclahealth.org/news/injectable-pre-exposure-prophylaxis-better-oral-form -
How Much Does Truvada for PrEPCost?. WebMD.
https://www.webmd.com/hiv-aids/how-much-truvada-for-prep-costs

By Yeva Margaryan, MD
Dr. Yeva Margaryan is a public health specialist specialized in population health promotion and research.

